- Online Book Store
- Wishlist
- Blogs
- Contact Us
- Login / Register
-
Subjects
- Agriculture
- Anthropology
- Architecture
- Ayurveda
- Biography
- Chemistry
- Children Books
- Commerce / Economics / Management
- Competition / Placement
- Computer
- Education
- Encyclopedia / Reference / General
- Engineering & Technology
- English Literature / Language
- Environmental Science
- Food & Home Science
- Geography
- Geology
- Hindi
- History
- Home Page Book
- Home Science
- Hospital Management
- Journalism / Mass Communication
- Journals
- Law
- Library & Information Science
- Life Sciences / Biotechnology
- Material Science
- Mathematics
- Medical/Nursing/Hospital/Pharmaceutical
- Physics
- Political Science
- Psychology
- Rare Books
- Religion
- Research Method
- Sanskrit / Indology
- School Book
- Social Work
- Sociology
- Tourism
- Water Science
- Women Studies
- Yoga / Sports / Health
- Yoga,
- Home
-
Subjects
- Agriculture
- Anthropology
- Architecture
- Ayurveda
- Biography
- Chemistry
- Children Books
- Commerce / Economics / Management
- Competition / Placement
- Computer
- Education
- Encyclopedia / Reference / General
- Engineering & Technology
- English Literature / Language
- Environmental Science
- Food & Home Science
- Geography
- Geology
- Hindi
- History
- Home Page Book
- Home Science
- Hospital Management
- Journalism / Mass Communication
- Journals
- Law
- Library & Information Science
- Life Sciences / Biotechnology
- Material Science
- Mathematics
- Medical/Nursing/Hospital/Pharmaceutical
- Physics
- Political Science
- Psychology
- Rare Books
- Religion
- Research Method
- Sanskrit / Indology
- School Book
- Social Work
- Sociology
- Tourism
- Water Science
- Women Studies
- Yoga / Sports / Health
- Yoga,
- About
- New Releases
- Catalogue
- Events
- Institution / Universities / Colleges
- Publish With Us
- Authors
- Home
-
Subjects
- Architecture
- Computer
- Anthropology
- Agriculture
- Ayurveda
- Chemistry
- Children Books
- Competition / Placement
- Commerce / Economics / Management
- Education
- Encyclopedia / Reference / General
- English Literature / Language
- Environmental Science
- Geography
- Geology
- Hindi
- Home Science
- Hospital Management
- History
- Journalism / Mass Communication
- Law
- Library & Information Science
- Life Sciences / Biotechnology
- Mathematics
- Physics
- Political Science
- Psychology
- Religion
- Research Method
- Sanskrit / Indology
- Sociology
- Social Work
- Women Studies
- Yoga / Sports / Health
- Home Page Book
- Rare Books
- Journals
- Yoga,
- Engineering & Technology
- Food & Home Science
- Material Science
- Water Science
- Medical/Nursing/Hospital/Pharmaceutical
- School Book
- Biography
- Tourism
- About
- New Releases
- Events
- Catalogues
- Institution / Universities / Colleges
- Publish With Us
- Authors
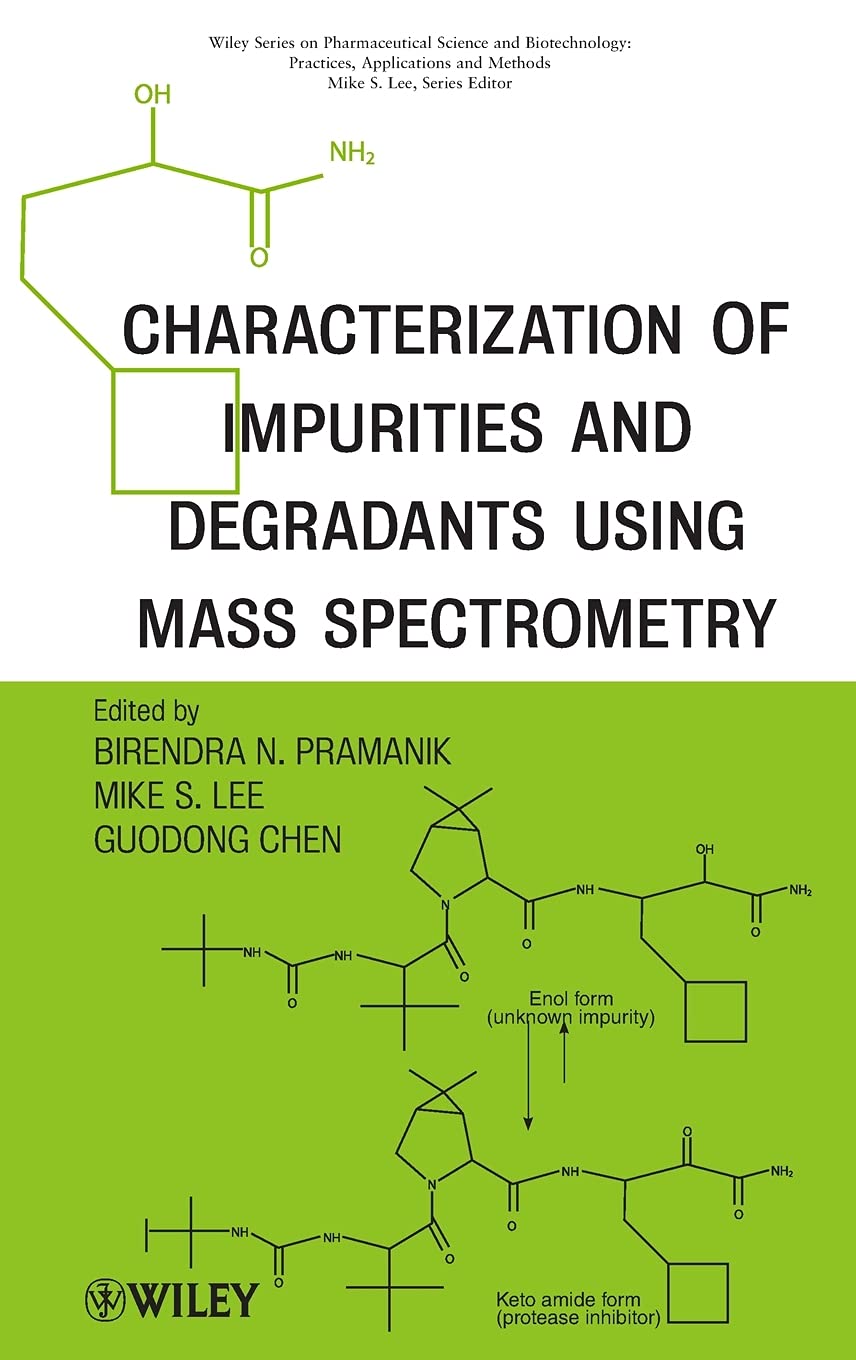
Characterization of Impurities and Degradants Using Mass Spectrometry
Characterization of Impurities and Degradants Using Mass Spectrometry
- Availability: In 1 Stock
- Be the first to review this product
About the book
The book highlights the current practices and future trends in structural characterization of impurities and degradants. It begins with an overview of mass spectrometry techniques as related to the analysis of impurities and degradants, followed by studies involving characterization of process related impurities (including potential genotoxic impurities), and excipient related impurities in formulated products. Both general practitioners in pharmaceutical research and specialists in analytical chemistry field will benefit from this book that will detail step-by-step approaches and new strategies to solve challenging problems related to pharmaceutical research.
Contents
PART I: METHODOLOGY
CHAPTER 1
Introduction to Mass Spectrometry (Pages: 1-57)
CHAPTER 2
LC Method Development and Strategies (Pages: 59-79)
CHAPTER 3
Rapid Analysis of Drug-Related Substances Using Desorption Electrospray Ionization and Direct Analysis in Real Time Ionization Mass Spectrometry (Pages: 81-108)
CHAPTER 4
Orbitrap High-Resolution Applications (Pages: 109-134)
CHAPTER 5
Structural Characterization of Impurities and Degradation Products in Pharmaceuticals Using High-Resolution LC-MS and Online Hydrogen/Deuterium Exchange Mass Spectrometry (Pages: 135-181)
CHAPTER 6
Isotope Patten Recognition on Molecular Formula Determination for Structural Identification of Impurities (Pages: 183-211)
PART II: APPLICATION
CHAPTER 7
Practical Application of Very High-Pressure Liquid Chromatography across the Pharmaceutical Development–Manufacturing Continuum (Pages: 213-229)
CHAPTER 8
Impurity Identification for Drug Substances (Pages: 231-250)
CHAPTER 9
Impurity Identification in Process Chemistry by Mass Spectrometry (Pages: 251-277)
CHAPTER 10
Structure Elucidation of Pharmaceutical Impurities and Degradants in Drug Formulation Development (Pages: 279-335)
CHAPTER 11
Investigation of Degradation Products and Extractables in Developing Topical OTC (Over the Counter) and NCE (New Chemical Entity) Consumer Healthcare Medication Products (Pages: 337-390)
CHAPTER 12
Characterization of Impurities and Degradants in Protein Therapeutics by Mass Spectrometry (Pages: 391-426)
CHAPTER 13
Identification and Quantification of Degradants and Impurities in Antibodies (Pages: 427-459)
| Title | Characterization of Impurities and Degradants Using Mass Spectrometry |
| Author | Mike S.Lee |
| ISBN | 9780470386187 |
| Publisher | Wiley |
| Pages | 462 |
| Binding | Hardcover |
No Tag(s).
Bought a Product, Please login & give your review !!
No Review(s).
Bought a Product, Please login & give your review !!